Abstract
Introduction:Hemophilia A is an inherited bleeding disorder characterized by deficiency of factor VIII resulting in recurrent and spontaneous musculoskeletal bleeding. Treatment-related challenges including development of inhibitors to FVIII, venous access problems, and pain at infusion site can impact a patient's quality of life and may subsequently affect patient satisfaction with treatment. Recent advances have extended the half-life of factor replacement therapy which can allow less frequent dosing. The health-related quality of life (HRQOL) and satisfaction with treatment are reported in children with severe hemophilia A who were treated with turoctocog alfa pegol, a novel recombinant FVIII product.
Methods: Children with severe hemophilia A aged 0-12 years who participated in the single-arm phase III pathfinder™5 trial were included in this analysis. In the main phase of the trial, 68 children were treated using 60 IU/kg turoctocog alfa pegol IV twice weekly for 26 weeks. HRQOL was assessed by children and their parents using age-specific versions of the Hemophilia-Quality of Life questionnaire (HAEMO-QOL-I for ages 4-7 and HAEMO-QOL-II for ages 8-12). Satisfaction was assessed by parents using the Hemophilia Satisfaction questionnaire (HEMO-SAT). HAEMO-QOL and HEMO-SAT scores range from 0 to 100, with a lower score reflecting a better QOL or higher treatment satisfaction. Changes in the HAEMO-QOL and HEMO-SAT scores from baseline to the end of the main phase of the trial were assessed only for those individuals who completed the questionnaires at both time points.
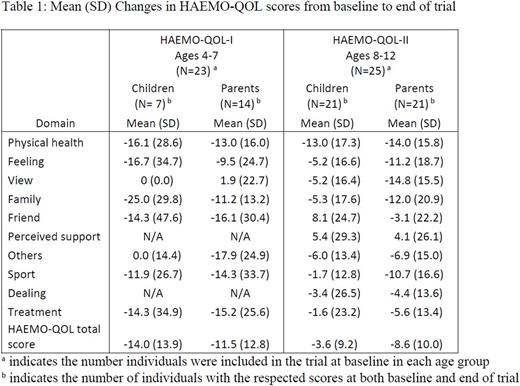
Results: Sixty-eight children participated in the trial (20 aged 0-3 years, 23 aged 4-7 years, 25 aged 8-12 years). Table 1 presents the change in scores of the individuals who completed the questionnaires at both time points in each age group. As reported by the children aged 4-7 and their parents, the mean HAEMO-QOL-I total score decreased (reflecting an improvement for the group) from baseline to end of the trial (29.6 to 22.3 for children; 31.2 to 20.5 for parents). Change in individual scores (N=7 children; N=14 for parents), reflected improvement of HRQOL in 6 out of the 8 domains and in the total score (mean change -14.0±13.9) for the children and in 7 out of the 8 domains and in the total score (mean change -11.5±12.8) for the parents. As reported by the children aged 8-12 and their parents, the mean HAEMO-QOL-II total score decreased (reflecting an improvement for the group) from baseline to end of the trial (21.7 to 17.9 for children; 29.2 to 20.4 for parents). Changes in individual scores (N=21 for children; N=21 for parents), reflected improvement of HRQOL in 8 out of the 10 domains and in the total score (mean change -3.6±9.2) for the children and in 9 out of the 10 domains and in the total score (mean change -8.6±10.0) for the parents. Parents reported low scores in all domains of the HEMO-SAT at both baseline (N=19 for aged 0-3 years, N=20 for aged 4-7 years, N=22 for aged 8-12 years) and end of main phase (N=16 for aged 0-3 years, N=18 for aged 4-7 years, N=21 for aged 8-12 years) indicating that they maintained a high level of satisfaction during the initial 26 weeks of treatment with turoctocog alfa pegol.
Conclusions:Overall, children with severe hemophilia A who were treated with turoctocog alfa pegol consistently reported improvement in HRQOL and maintained a high level of satisfaction by the end of the 26-week main phase of the trial.
Kearney: Bioverativ: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Grifols: Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Pham: Novo Nordisk: Consultancy. Adalsteinsson: Novo Nordisk: Employment. Landorph: Novo Nordisk: Employment. Raffini: CSL Behring: Consultancy; Genetech: Consultancy; Green Cross Inc: Consultancy; Bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal